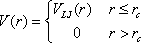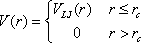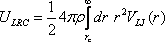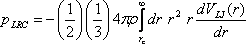Benchmark results for Lennard-Jones fluid
This website is best viewed with younger browsers
(for example: Netscape 7, IE 6, Opera 7, Firefox 1)
The purpose of these pages is to provide some explicit results from
molecular dynamics and Monte Carlo simulations for the Lennard-Jones
fluid. It is intended to provide guides for testing codes. Reproducing
these results is a test of the correctness of codes, either written by
the user or obtained elsewhere. The explicit conditions for each of the
sets of results are supplied so that meaningful comparisons of your
results with the ones listed here are possible.
The information presented here has been organized into
six
different pages. It is available as web pages or as a single
excel file.
- MD:
NVE Molecular dynamics results at liquid-like densities
along an isotherm of reduced temperature 0.85. Mean values and standard
deviations of temperature, energy, pressure, and diffusion coefficient
are reported.
- MC:
NVT Monte Carlo results at both liquid- and vapor-like
densities along two isotherms of reduced temperature 0.85 and 0.90.
Mean values and standard deviations of energy and pressure are
reported.
- SAT-TMMC:
Liquid-vapor coexistence properties obtained by grand-canonical
transition-matrix Monte Carlo and histogram re-weighting [1] over the reduced temperature range 0.70 to 1.20
at increments of 0.05. Mean values and standard deviations of the
saturation pressure and coexisting liquid and vapor densities, energies, and activities are
reported.
- EOS-TMMC:
Equations of state (pressure as a function density) generated by
grand-canonical transition-matrix Monte Carlo over the temperature
range 0.70 to 1.20 and 1.35 - 1.50 at
increments of 0.05. For a given density, the mean
pressure and its standard deviation are reported. CPU timings and
downloadable raw simulation data (particle number probability
distributions) are provided here.
- SAT-EOS:
Liquid-vapor coexistence properties as determined from an empirical fit
of a large amount of simulation data [2]. The data
serve as an approximate guide for liquid-vapor coexistence properties
at temperatures other than those investigated in Item 3. Furthermore,
the empirical fit should not be used in the vicinity of the critical
point.
- SURFACE TENSION:
Surface tension of the Lennard-Jones fluid at various temperatures calculated using explicit
molecular dynamics of the liquid-vapor interface and the combination of finite-size scaling and grand-canonical transition-matrix Monte Carlo. The influence
of truncation is also investigated in this section.
- SAT-TMMC:
Liquid-vapor coexistence properties for the Lennard-Jones fluid, linear-force shifted at r=2.5σ obtained by grand-canonical
transition-matrix Monte Carlo and histogram re-weighting [1] over the reduced temperature range 0.65 to 0.90
at increments of 0.05. Mean values and standard deviations of the
saturation pressure and coexisting liquid and vapor densities, energies, and activities are
reported.
- VAPOR-EOS-TMMC:
Vapor-phase properties for the Lennard-Jones fluid, linear-force shifted at r=2.5σ obtained by grand-canonical
transition-matrix Monte Carlo and histogram re-weighting [1] over the reduced temperature range 0.65 to 0.90
at increments of 0.05. Mean values and standard deviations of the
saturation pressure and coexisting liquid and vapor densities, energies, and activities are
reported.
As is usually the case, temperature, density (number density),
pressure, etc., are given in reduced units (denoted by *). That is,
these properties are expressed in terms of the characteristic energy,
ε, and length scale, σ, defined by the Lennard-Jones potential:
VLJ(r) = 4 ε [ (σ/r)12 - (σ/r)6]
Therefore, the reduced temperature T*, density ρ*, and pressure p*
are kBT/ε, ρσ3, pσ3/ε, respectively.
The critical parameters for the pure LJ fluid have been determined to
be Tc* = 1.3120(7), ρc* = 0.316(1), and pc*
= 0.1279(6) [3].
For computational expediency, the potential and force are usually
truncated at some cutoff distance rc. That is, the effective
potential
V(r) is
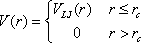
In this work, the cutoff distance is taken to be 3σ,
unless noted otherwise. An approximate
correction for this truncation to the energy and pressure of the system
can be obtained by assuming the spatial correlations beyond the cutoff
distance are unity. The reader is referred to Refs. [4]
and [5] for these so-called “standard
long range
corrections” (sLRC). It should be noted that this not a good assumption
in inhomogeneous fluids. For completeness, the working expressions for
obtaining the long range corrections to the potential energy per
particle and virial pressure are provided below:
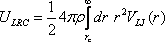
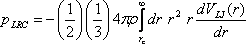
where ρ is the bulk number
density, VLJ(r)
is the Lennard-Jones potential energy, and rc
is the truncation (cutoff) distance. A subtle point to note is that the
above pressure correction does not account for impulsiive effects at rc
where the potential energy (and therefore the force) changes
discontinuously. This is particularly important when trying to simulate
directly interfacacial phenomena [6]. Finally, it
should be mentioned that other truncation and long range correction
schemes for dealing with non-bonded interactions exist [4-8]. In some
cases, the truncation schemes can yield critical properties that differ
from those quoted above [8].
In future versions, coordinate sets will be available for which the
potential energy per particle and the virial are specified. This
information can be used to test energy/force routines.
References
[1] J. R. Errington, J. Chem. Phys. 118,
9915 (2003).
[2] J. K. Johnson, J. A. Zollweg, and K. E. Gubbins,
Mol. Phys. 78, 591
(1993).
[3] J. J. Potoff and A. Z. Panagiotopoulos, J.
Chem. Phys. 109, 10914
(1998)
[4] M. P. Allen and D. J. Tildesley, Computer
Simulation of Liquids (Oxford University Press, New York, 1989).
[5] D. Frenkel and B. Smit, Understanding
Molecular Simulation, 2nd ed. (Academic, San Diego, 2002).,
pp.37-38.
[6] A. Trkohymchuk and J. Alejandre, J.
Chem. Phys. 111, 8510
(1999).
[7] D. N. Theodorou and U. W. Suter, J. Chem. Phys. 82, 955 (1985).
[8] B. Smit, J. Chem. Phys.
96, 8639 (1992).