| METHOD: | Grand-canonical transition-matrix Monte Carlo [1] and histogram re-weighting |
| V/σ3: | 512 |
| TRUNCATION: | Linear Force Shifted at 2.5σ |
| Prob. of Disp. Move: | 0.40 |
| Prob, of Ins/Del. Move: | 0.60 |
| Biasing Function Update
Frequency |
1.0E6 trials |
| Simulation Length: | 8.0E9 trials |
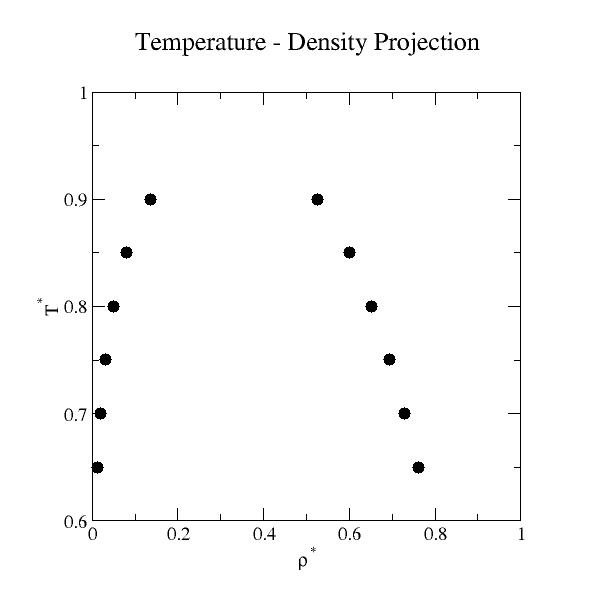
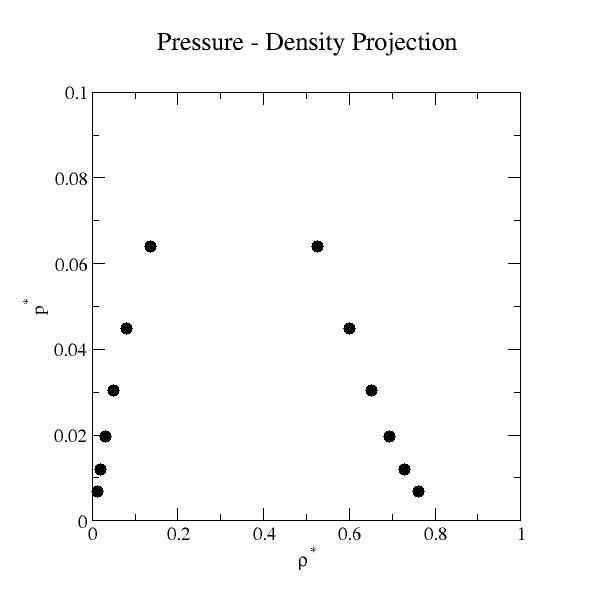
Liquid-Vapor Phase Coexistence Properties:
| T* | ρvap* |
+/- | ρliq* | +/- | psat* | +/- | Uvap* | +/- | Uliq* | +/- | ln z |
+/- |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.65 | 1.13086E-02 | 2.38587E-06 | 7.61685E-01 | 1.75209E-04 | 6.71314E-03 | 1.28036E-06 | -1.11676E-01 | 2.44461E-05 | -4.21546E+00 | 1.80378E-03 | -4.65535E+00 | 1.74181E-04 |
| 0.70 | 1.95113E-02 | 1.67304E-06 | 7.29309E-01 | 1.93161E-04 | 1.18969E-02 | 8.67525E-07 | -1.80182E-01 | 1.61809E-05 | -3.99813E+00 | 4.68222E-04 | -4.19453E+00 | 6.35176E-05 |
| 0.75 | 3.18771E-02 | 4.34360E-06 | 6.93338E-01 | 8.77657E-05 | 1.95383E-02 | 2.06799E-06 | -2.77685E-01 | 3.98990E-05 | -3.76690E+00 | 7.03921E-04 | -3.81176E+00 | 8.64963E-05 |
| 0.80 | 5.04378E-02 | 1.23324E-05 | 6.52056E-01 | 1.51115E-04 | 3.02550E-02 | 4.98952E-06 | -4.16314E-01 | 1.07693E-04 | -3.51491E+00 | 7.52792E-04 | -3.49051E+00 | 1.23641E-04 |
| 0.85 | 7.95146E-02 | 1.37074E-05 | 6.01040E-01 | 1.36261E-04 | 4.47490E-02 | 4.00928E-06 | -6.23068E-01 | 1.14899E-04 | -3.22499E+00 | 6.42761E-04 | -3.21839E+00 | 5.93251E-05 |
| 0.90 | 1.34982E-01 | 3.46267E-05 | 5.24430E-01 | 2.05138E-04 | 6.39805E-02 | 4.35001E-06 | -1.00685E+00 | 2.75183E-04 | -2.84264E+00 | 9.86622E-04 | -2.98636E+00 | 3.58074E-05 |
Remarks:
Uncertainties were obtained
from 5 independent simulations and represent 95% confidence limits based on a standard t statistic.
Liquid-vapor coexistence was determined by adjusting the activity such
that the pressures of the liquid and vapor phases were
equal. Here, the pressure is not the conventional virial pressure [2,
3] but is the actual thermodynamic pressure, based on the
fact that the absolute free energies can be obtained from the
distributions determined from simulation [4]. Alternative methods, for
example Gibbs-ensemble Monte Carlo and combination grand-canonical
Monte Carlo and histogram re-weighting, can be used to determine
liquid-vapor coexistence. A review of standard methods of phase
equilibria simulations can be found in Ref. 5.
As introduced in Refs. 2 and 3, the activity, z, is defined as
z = Λ-3 exp(βμ)
where Λ is the de Broglie wavelength, β = 1/(kBT)
(where kB is Boltzmann's constant),
and μ is the chemical potential. It is sometimes more convenient to
work with ln z in the simulations and in post-processing. (NOTE: The reported activity is dimensionless, having been scaled by the LJ length cubed.)
Phase-coexistence energies were obtained by determining the
mean potential energy at a given value of N for an additional 8 billion
MC trials. Combining this information with the particle number
probability distribution, the mean potential energy of the coexisting
phases can be calculated [6].
For the Lennard-Jones fluid, linear force shifted at 2.5σ, the critical properties are Tc*=0.937, ρc*=0.320, and pc*=0.0820 [7].
[1] J. R. Errington, J. Chem. Phys. 118,
9915 (2003).
[2] M. P. Allen and D. J. Tildesley, Computer
Simulation of Liquids
(Oxford University Press, New York, 1989).
[3] D. Frenkel and B. Smit, Understanding
Molecular Simulation, 2nd ed.
(Academic, San Diego, 2002).
[4] J. R. Errington and A. Z. Panagiotopoulos,
J. Chem. Phys. 109, 1093
(1998).
[5] A. Z. Panagiotopoulos, J. Phys.: Condens.
Matter 12, 25 (2000).
[6] J. R. Errington and V. K. Shen, J. Chem. Phys. 123, 164103 (2005).
[7] J. R. Errington P. G. Debenedetti, and S. Torquato, J. Chem. Phys. 118, 2256 (2003).